
(The electrons in the second energy level are the valence electrons).
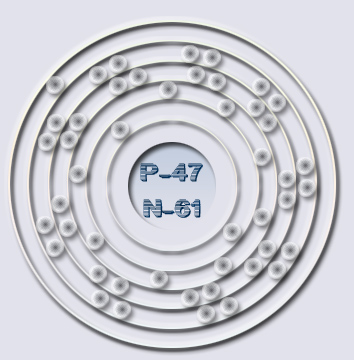
The Silver Atom - 5 Energy Levels - 47 Electrons/Protons

The third energy level has a capacity to accommodate eighteen electrons and

Electrons and energy | Back to Top
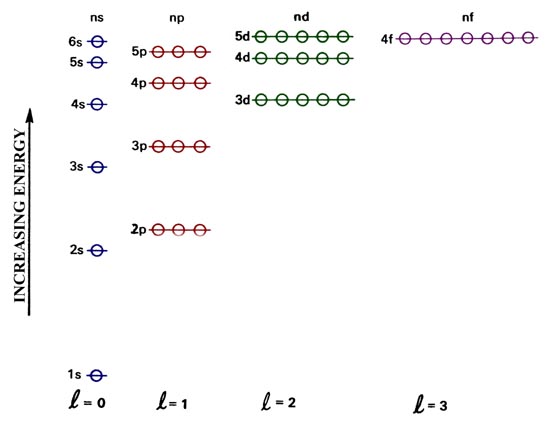
An orbital energy level diagram for a many-electron atom.

Notice that the fill order matches the energy levels

Electron Orbitals-Energy Levels-Shells-Subshells. Atom Structure-Electrons
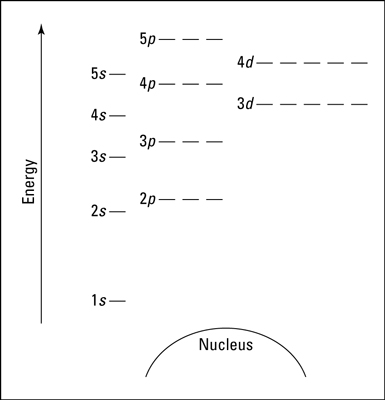
A blank energy level diagram. In the preceding diagram, orbitals are

The lowest energy level for electrons has a spherical shape and is referred

Electrons fill the lowest energy level first this means it is generally easy
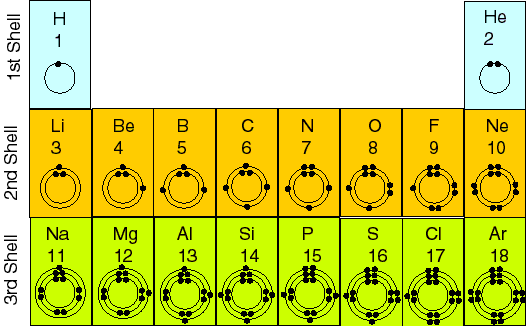
Here, the energy levels are called "shells."

An ELECTRON'S ENERGY LEVEL is the amount of energy required by an electron

Electrons in the inner energy levels also produce a screening effect.

Energy levels of electrons in an atom are quantized (discrete levels)

temperatures the energy levels are randomized and some electrons have
and how many electrons in each energy level.

Since each existing orbit has a unique radius and energy level, the photons

"energy level" is a vanishingly small energy interval of width d E d E .

Electrons In Energy Levels

Electrons in Definite energy Levels around the nucleus
